Most of you have heard of it (some of you might have even used it). It has gone by the name NutraSweet, Equal, and Sugar Twin, but the majority of people know it by the name associated with controversy and (supposedly) countless adverse health effects: aspartame. This product is an artificial, non-nutritive sweetener – “non-nutritive” insinuates that there are very few calories (if any) in the product. It is used in diet sodas and foods that are “sugar-free.” Additionally, it has been approved to be safe for human consumption by the Food and Drug Administration (FDA). Unfortunately, there have been people (horrible individuals, really) who have spread lies about aspartame. So, without further ado, let’s get into the real science behind this “dangerous” sweetener.
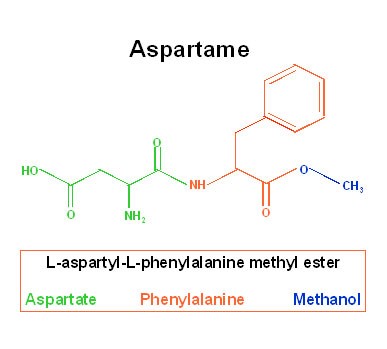
First off, to understand the concept that this sweetener is truly safe, you need to become a little bit familiar with the chemical structure of aspartame. There are three components of aspartame: aspartate (an amino acid that is also known as aspartic acid), phenylalanine (another amino acid), and methanol (also called methyl alcohol). Now, by themselves, are any of these components toxic? The answer is NO (well…for most people they’re not – look at the second-to-last paragraph for my input on that)! Why do I say this? To answer that question, let’s delve a bit deeper into aspartame’s components.
Aspartate and phenylalanine are two of many amino acids – the building blocks of protein. They are actually found in foods that are relatively common to our everyday diets. Both aspartate and phenylalanine, for example, have fairly high amounts in chicken and skim milk. In about 3.5 ounces of roasted, boneless, skinless chicken breast, there is 1231 mg phenylalanine and 2764 mg of aspartate. In 12 ounces of a 100% aspartame-sweetened soda, there is roughly 90 mg of phenylalanine and 72 mg of aspartate – those quantities are significantly less than the ones found in chicken. Now…I don’t know about you, but I have never suffered from any adverse health effects related to the phenylalanine and aspartate content of 3.5 ounces of chicken. I know I’ve eaten a lot more than 3.5 ounces of chicken in one meal! What you need to remember: whether the aspartate or phenylalanine are in a piece of roasted chicken or an aspartame-sweetened beverage, the amino acids are metabolized (broken down and used by our body) the exact same way. Our bodies don’t see them as different.
Next we have methanol (yes, it is chemically classified as an alcohol…do not try to get drunk off of this stuff). This component of aspartame is the “toxic” portion, if you wanted to get really technical. It is true that 2 tablespoons (about 1 ounce) of methanol could kill a child, and it is also true that 2 to 8 ounces could kill an adult (NLM, 2015); HOWEVER, those specific amounts of methanol will never be present in foods (don’t get me started on cross-contamination…that’s for a different article). Let’s go back to our 12 ounce, 100% aspartame-sweetened soda – in this beverage, there is about 18 milligrams of methanol. Okay, cool…now, let’s look at 12 ounces of tomato juice: there’s roughly 107 milligrams of methanol! By some mathematical conversions, I was able to figure out how much 107 milligrams translated to in ounces… it translates to approximately 0.004 ounces. So, even if you drank 12 ounces of 100% aspartame-sweetened soda, you have to remember that it has less than 20% of the amount of methanol in 12 ounces of tomato juice, AND even the tomato juice contains 0.4% of the toxic level of methanol for a child. The basic point: methanol is present naturally in produce and products of produce – its content in these foods is typically greater than in foods sweetened by aspartame.
Lastly, aspartame, as a sweetener, is EXTREMELY sweet. It is actually sweeter than sucrose (a science-y name for sugar); according to the Food and Drug Administration, “it is about 200 times sweeter than table sugar” (2015). That also leads to another reason why you’ll never have adverse health effects from this product (or the foods sweetened by it): since it is sweeter than sugar, you would need a very, very miniscule amount of aspartame to sweeten something. Let me give an example: imagine that we were making a huge batch of lemonade, and we figured out that we needed 12.5 cups of sugar (sucrose). There’s a small problem, though… When we go to our pantry, all we can find is a box of aspartame packets; however, there’s a conversion chart, and we realize that all we need is, roughly, 1 tablespoon of aspartame to substitute for the 12.5 cups of sugar. That’s a really, really, really big difference in amounts, but because aspartame is so much sweeter than sucrose, those values are a reality.
According to the FDA’s website, “FDA scientists have reviewed scientific data regarding the safety of aspartame in food and concluded that it is safe for the general population under certain conditions” (2015). What are those certain conditions? There is a disease called phenylketonuria (PKU); the individuals who have this disease are not able to process the amino acid phenylalanine correctly, due to a flaw in a metabolic pathway. Because of this factor, consumption of aspartame could mean a bad health-related experience for people with PKU. Hence, this is the one group of individuals that is instructed not to consume products sweetened with aspartame.
There you have it. I’ve given my input on aspartame, and I’ve hopefully explained the science behind the sweetener’s safety. I understand that some people are still wary of the whole idea behind artificial sweeteners, but it’s important to realize that they are, in no way, capable of causing horrible health effects to the human population. Their components (specifically the two amino acids) are metabolized in the body in the exact same way as if they came from “natural” foods. Do keep in mind that I am not trying to push my own perspectives on anyone. All I’d like for you to consider is this: understand the science before you fear the food!
- This is the FDA’s webpage that I cited within my article: http://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm397725.htm
- Here’s the U.S. National Library of Medicine’s article that I cited: https://www.nlm.nih.gov/medlineplus/ency/article/002680.htm
- This is what the American Cancer Society has to say about aspartame: http://www.cancer.org/cancer/cancercauses/othercarcinogens/athome/aspartame
- Here’s the resource I used to figure out the phenylalanine and aspartate content of foods: http://nutritiondata.self.com/
- Examples and comparisons of food products adapted from Dr. Bonny Burns-Whitmore’s Advanced Nutrient Metabolism Lecture Powerpoint: “Basic Structure, Digestion, Synthesis [of Proteins].” Slides 74-76. 2015.
- Picture credit: Dr. Bonny Burns-Whitmore’s Advanced Nutrient Metabolism Powerpoint: “Basic Structure, Digestion, Synthesis [of Proteins].” Slide 72. 2015.
